Aqueous zinc-ion batteries (AZIBs) are considered one of the most promising safe energy storage technologies due to their high safety, high theoretical capacity, low cost, and environmental friendliness.However, undesirable side reactions on the Zn anode surface, such as dendrite growth and hydrogen evolution corrosion, can lead to the premature failure of AZIBs. Therefore, it is crucial to develop long-life Zn-metal anodes. Electrolyte engineering is an effective strategy to stabilize Zn-metal anodes. However, a single solute or solvent additive is far from sufficient to meet the requirements for electrolyte cycling stability. Therefore, it is essential to identify approaches that leverage the synergistic effects of multiple components to fully exploit the performance of individual additives and effectively extend the cycling lifespan of Zn-metal anodes.
In light of this, Professor Yagang Yao's team at Nanjing University designed a new-type high-entropy electrolyte composed of equal molar amounts of Zn(OTf)2 and LiOTf, along with equal volumes of H2O, triethyl phosphate, and dimethyl sulfoxide, which enhances electrolyte stability by increasing solvation entropy. The organic solvents TEP and DMSO, which possess high donor numbers (DNs), exhibit strong solvation capabilities that allow them to replace water molecules in the solvation shell of Zn2+ and suppress the activity of free water through the reconstruction of the hydrogen-bond network. Simultaneously, the dynamic electrostatic shielding effect of Li+ promotes a uniform distribution of the interfacial electric field, facilitating even adsorption and deposition of Zn2+ on the anode surface, and ultimately preventing the growth of Zn dendrites. Additionally, the in situ decomposition of OTf-, TEP, and DMSO on the Zn surface leads to the formation of a gradient solid-electrolyte interphase (SEI) layer enriched with inorganic components, which not only facilitates the rapid transport of Zn²⁺ but also prevents the continuous decomposition of water on the electrode surface and alleviates electrode corrosion, and suppresses dendrite growth by withstanding external stress. Consequently, the resulting Zn//Zn symmetric cell using this high-entropy electrolyte can operate over 8000 h at 1 mA cm-2 and 0.5 mAh cm-2. The resultingZn//V2O5·H2O pouch cell retains 83.1% of its capacity after 420 cycles at 1 A g-1. This work leverages the prominent synergistic effects of different components to achieve greater disorder and complexity, offering important reference for optimizing the Zn/electrolyte interphase and enhancing the stability of Zn-metal anodes.
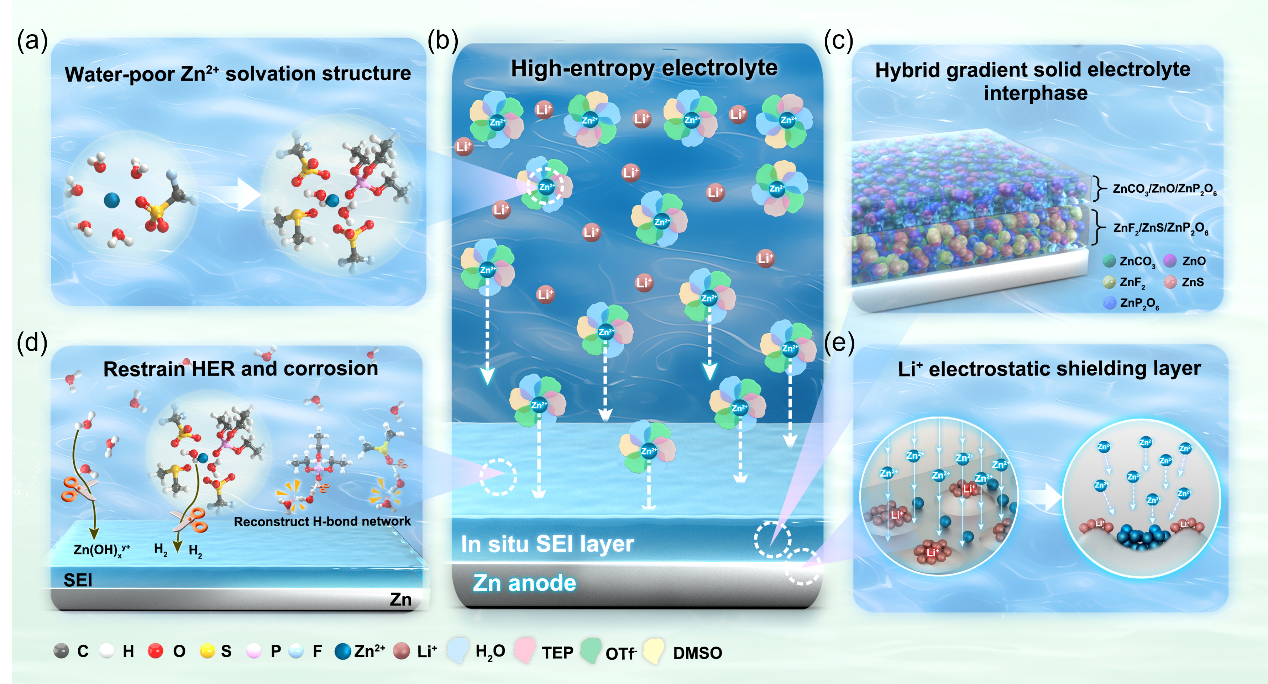
Figure 1. (a) Solvation structure of the high-entropy electrolyte. (b) Principle and underlying mechanism of the high-entropy electrolyte. (c) Main components of the SEI. (d) Inhibition of hydrogen evolution reactions and corrosion. (e) Electrostatic shielding mechanism of Li+.
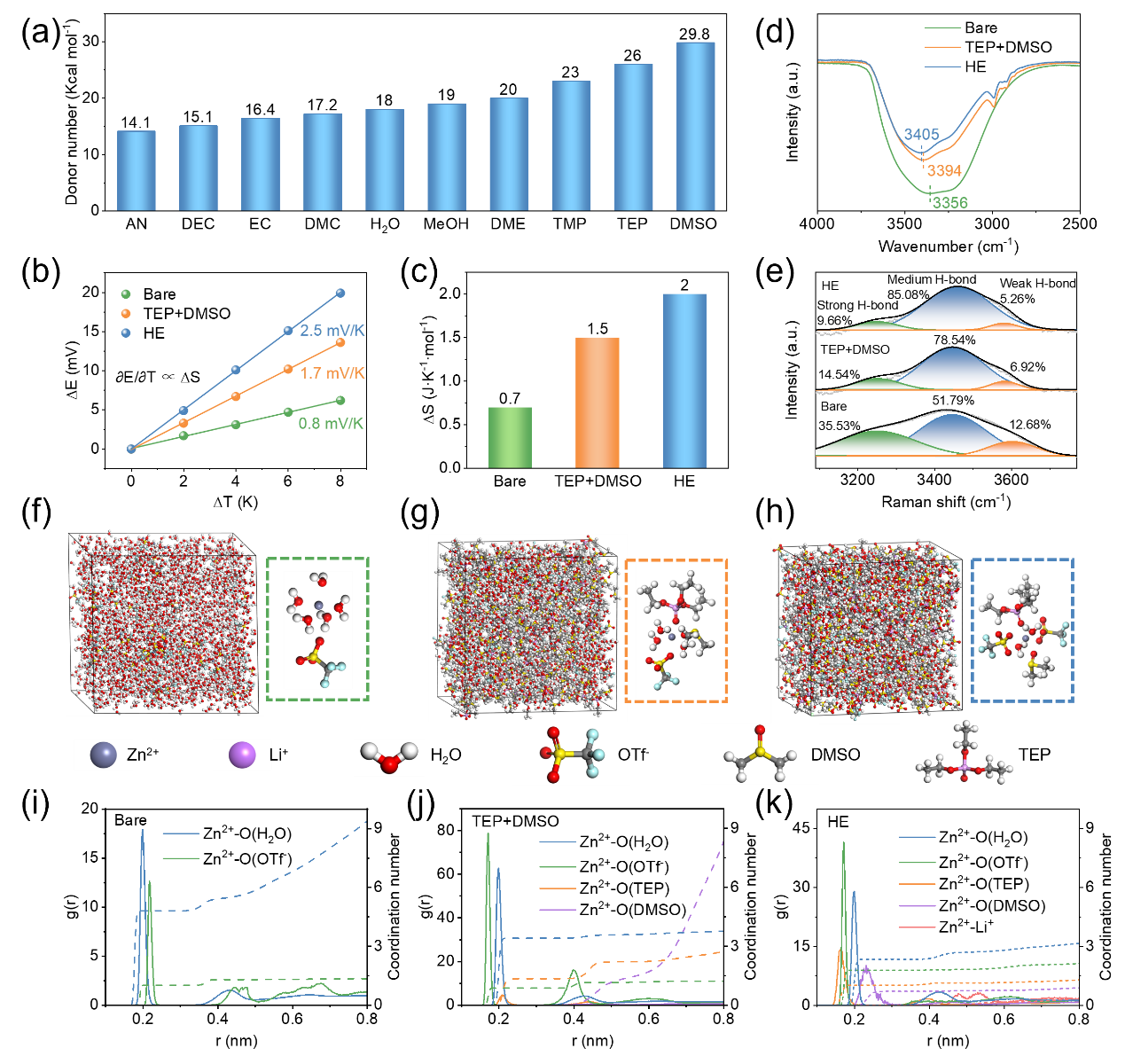
Figure 2.(a) Donor numbers of various common solvents. Entropy changes in different electrolyte systems, derived based on (b) experiment and (c) theoretical calculations. (d) FTIR and (e) Raman spectra of O-H stretching vibrations in different electrolytes. 3D snapshot of (f) bare, (g) TEP+DMSO, and (h) HE electrolytes and the corresponding solvation structure of Zn2+ obtained from MD simulations. Radial distribution functions and coordination number distribution functions obtained from MD simulations of (i) bare, (j) TEP+DMSO, and (k) HE electrolytes.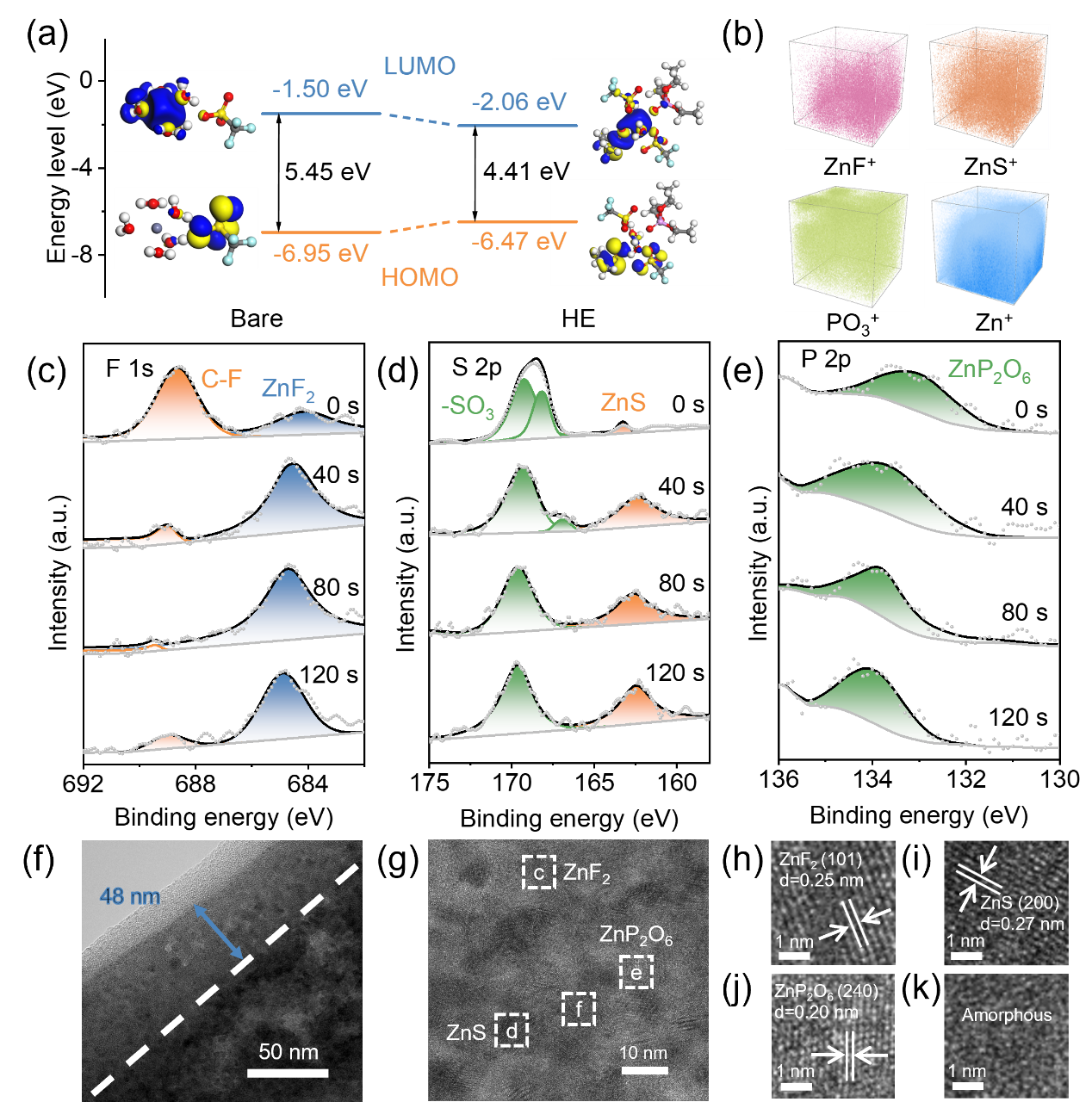
Figure 3. (a) LUMO and HOMO energy levels of solvation structures in bare and HE electrolytes. (b) TOF-SIMS 3D spectra of ZnF+, ZnS+, PO3+, and Zn+ in HE electrolyte. In-depth XPS spectra of (c) F 1s, (d) S 2p, and (e) P 2p on the Zn anode surface after 30 cycles in HE electrolyte. (f) TEM and (g-k) HRTEM images of Zn anode after 30 cycles in HE electrolyte.
This work, titled “High-Entropy Aqueous Electrolyte Induced Formation of Water-Poor Zn2+ Solvation Structures and Gradient Solid-Electrolyte Interphase for Long-Life Zn-Metal Anodes,” was published in “Angewandte Chemie International Edition”, a leading journal in the field of materials science. Lin Lin, Ph.D. student at College of Engineering and Applied Sciences at Nanjing University, is the first author of the paper. This research received substantial assistance from the National Laboratory of Solid State Microstructures, Jiangsu Key Laboratory of Artificial Functional Materials and Collaborative Innovation Center of Advanced Microstructures
Article Link: https://doi.org/10.1002/anie.202425008

